Stringent Quality Control for Optimal Product Performance
We operate in a strictly controlled production environment with a comprehensive testing process. Our highly trained quality control personnel, supported by detailed data analytics, ensure our products meet various physical standards such as particle content, microbial limits, leakage resistance, tensile strength, blasting strength, and other regulatory requirements for bacterial packaging. For specific packaging needs, additional tests are performed based on the material’s nature, intended use, and applicable regulatory standards.
Why Choose Hopeway AMD Group for Your Sterilization Packaging Needs?
As a Hopeway AMD customer, you can trust that our sterilization packaging solutions meet the highest standards of quality, safety, and compliance, ensuring your products are always fully protected.
Hopeway AMD Group Compliance with Key International Standards:

- TÜV SÜD certified EN ISO13485:2016, Medical device – Quality Management System
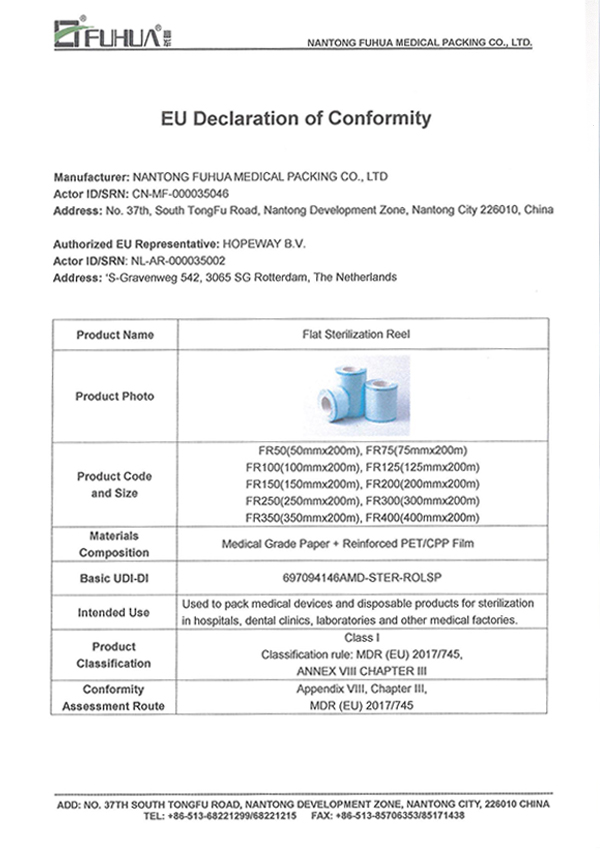
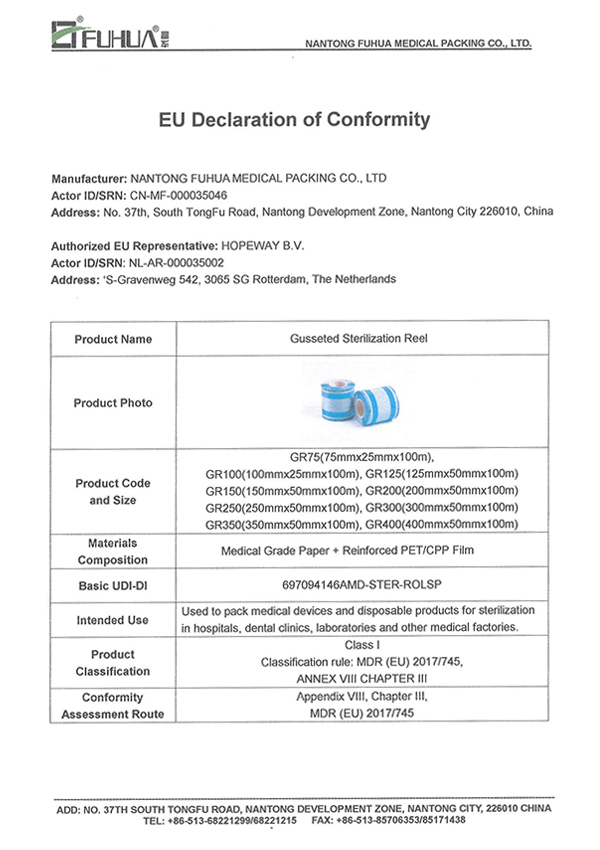
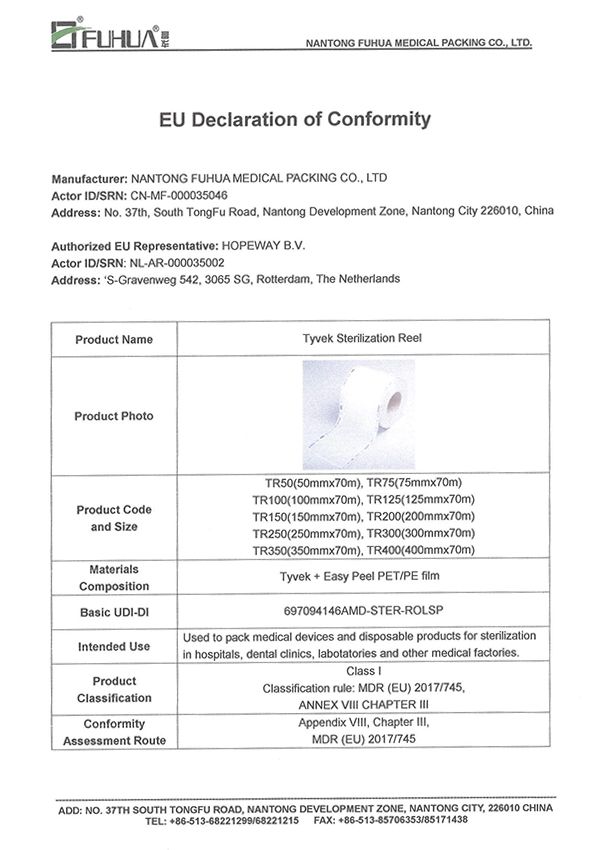
MDR(EU) 2017/745
EN 868
EN ISO 11607
EN ISO 11140

 English
English Français
Français Deutsch
Deutsch Nederlands
Nederlands






















 ‘s-Gravenweg 542, 3065SG Rotterdam
‘s-Gravenweg 542, 3065SG Rotterdam +31 (0)10 254 28 08
+31 (0)10 254 28 08 info@hopewayamd.com
info@hopewayamd.com






